“The expert angle” Let’s Talk Sarcoma…
Ben Fordham and Wippa – 2GB
Press the link below to hear the full version of the 2GB interview with Ben Fordham and Wippa, who shine the spotlight on sarcoma, and the Let’s Talk Sarcoma podcast series.
Cooper Rice-Brading. His dying wish…
AYA’s with cancer webinar series
Cancer Nurses Society of Australia are conducting a series of webinars for Adolescents and young adults living with cancer.
Tuesday 11 August: An introduction to cancer in the AYA population
Tuesday 8 September: Special considerations when working with AYAs with cancer
Tuesday 6 October: Discussing fertility, sexual health and other sensitive topics with AYAs with cancer. To register for these webinars, press the link below:
RCA interview Tania Rice-Brading
Sock it to Sarcoma! WA Shines a light…
Perth based sarcoma not for profit, Sock it to Sarcoma has recently completed the fourth highly successful year of their “Shine a light” on sarcoma light show.
June 29 to July 5, marked Sarcoma Awareness Week in Western Australia, and specially chosen landmarks around the metropolitan areas and regional centres of WA landmarks were used to Shine a Light On Sarcoma. – by lighting up in the colours of Sock it to sarcoma (SITS!).
WA Sarcoma Awareness week each year sees Sock it to sarcoma! create a lasting community awareness for this sinister cancer, with this spectacular light show, and in doing so, potentially saving lives.
To learn more about the outstanding work being done by SITS! please visit their website at https://www.sockittosarcoma.org.au/

East coast billboard campaign
We are live!
Earlier this week, the CRBF #sarcomafootprint digital billboards launched at multiple sites in Brisbane, Melbourne, Gold Coast, and Sydney!
We have taken our awareness campaign, during the heart of sarcoma awareness month, to the streets of key Australian cities, thanks to the generosity of @QMS_media who donated this campaign in loving memory of Daniel Allchin, who lost his life to sarcoma in 2018.
We extend our gratitude to the remarkable team at QMS media, the wonderful Victoria Ellison, and the inspiring Allchin family, who each made this campaign possible. Also, huge thanks to Jordan Laing for putting the #sarcomafootprint campaign together, and to @jludemanncreative for designing the billboards.
Keep your eyes peeled when you’re driving around!
CRBF Sarcoma Awareness Month
Sarcoma Awareness Month
CRBF Press Release
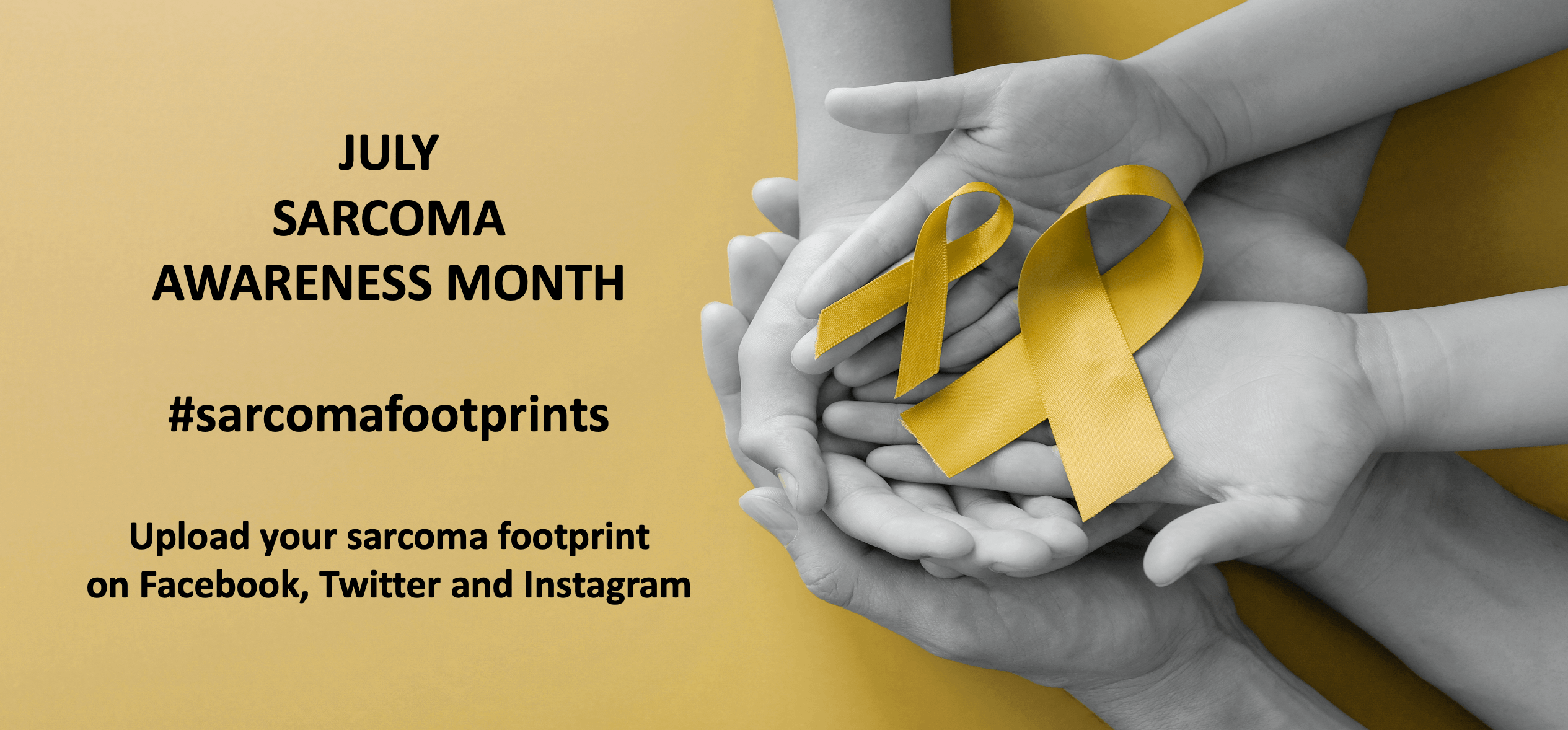
July is Sarcoma Awareness Month, and the Cooper Rice-Brading Foundation (CRBF) has once again joined the global initiative to raise awareness of sarcoma, a cancer that sadly shatters the lives of far too many patients and families.
Each year, CRBF funds innovative research leading to a cure, and supports sarcoma patients from diagnosis throughout treatment and beyond. Throughout July, the Foundation’s efforts to spread sarcoma awareness are accelerated.
Sarcoma can affect all ages, but hits our young disproportionately hard. Of all childhood cancers, one in five is a sarcoma, and survival outcomes remain amongst the worst of all childhood cancers. Yet, sarcoma receives less than one per cent of total cancer funding.
This July, the Foundation is calling upon anyone impacted by sarcoma, to upload the #sarcomafootprint image to social media, which can be found on the CRBF Instagram (@crbfoundation) and Facebook pages, to highlight sarcoma’s devastating footprint.
With each step forward, with time, and with carefully targeted funding and research, we can all fight sarcoma together and help to find a cure.
CRBF Chairman, Robert Beech-Jones said the Foundation is hoping to spread an important message during Sarcoma Awareness Month.
“It’s been an incredibly tough start to the year for all Australians, however, for some it’s been made even worse with the sarcoma diagnosis of a family member or friend. or the devastating passing of a loved one,” Beech-Jones said.
“Despite the outstanding efforts of many in the sarcoma community, survival outcomes and treatment options have not changed significantly in almost 40 years, while other cancers are making important inroads into finding a cure.
“Through more funding and research, we’re aiming to facilitate an eventual cure for sarcoma and to stop lives being cut short.
“We understand how difficult 2020 has been for most, but unfortunately cancer doesn’t slow during times of hardship, and each day more young people are being diagnosed with sarcoma.
“We’re hoping we can continue to help raise awareness of sarcoma, and to ensure more people are informed of its prevalence and severity.
“Please join us in using #sarcomafootprint this July by uploading a photo of the CRBF footprint, if you or someone you know has been impacted by sarcoma.”
For those living in Sydney, Melbourne, Adelaide, Brisbane and Gold Coast, keep an eye out for CRBF digital billboards on major roads promoting sarcoma awareness messages from July 6. The billboards were kindly donated to CRBF by QMS media, on behalf of Daniel’s Race for a Cure conducted by the Alchin family, in memory of Daniel Alchin.
To launch Global Sarcoma Awareness Month, CRBF, together with their West Australian counterpart, Sock it to Sarcoma!, have worked collaboratively to produce the podcast series: Let’s Talk About Sarcoma. Hosted by Cathrine Mahoney and Michael ‘Wippa’ Wipfli, the podcast will be available through Apple podcasts, or at crbf.org.au, with episodes set to be released on July 3. This series will cover the expected, the unexpected and everything in between, of a sarcoma diagnosis. Patients, families, clinicians and researchers will provide the raw and honest narrative.
Cooper Rice-Brading sadly lost his brave fight against bone cancer in 2017, just 18 months after being diagnosed with osteosarcoma.
Prior to his passing, Cooper incepted the Cooper Rice-Brading Foundation to raise much needed funding for research into sarcoma, with the vision of improved survival outcomes and treatment options for all sarcoma patients.
‘Let’s Talk About Sarcoma’ Podcast
In a collaboration to mark global awareness month, Sock it to Sarcoma and the Cooper Rice-Brading Foundation will host a series of free podcasts, to provide sarcoma patients and their families with a reliable resource from diagnosis to treatment and beyond. This series will be hosted by popular Sydney media identity, Michael ‘Wippa’ Wipfli and seasoned podcast presenter Cathrine Mahoney, creator of “So I quit my day job”.
In episode one we discuss how Sock it to Sarcoma and CRBF were incepted, and why, the need for what we do, our aims, and the current research we are funding. Special guest for the episode will be Dr Densie Caruso, CEO of the Australia New Zealand Sarcoma Association, discussing sarcoma. What it is, symptoms, and the importance of seeking professional advice, via a sarcoma specialist multi-disciplinary team.
Episode two will be broken into three parts, and will see patients from a cross section of sexes, age groups, and differing sarcoma subtypes, speaking about their experience from the moment they were symptomatic, diagnosis, treatment and beyond. This will be a raw account of each patient’s journey, with a special section on fertility and survivorship.
Episode three will see the spotlight shone on the families, friends, siblings and those who have tragically lost a loved one to sarcoma. This will provide a sensitive insight into managing the family dynamic leading up to diagnosis, throughout treatment and beyond. It will also explore grief, and the dire impact of losing a loved one to this cancer.
Episode four sees us finishing on a very positive note, when we hear from our leading researchers, clinicians, and a psycho-social researcher, covering a myriad of topics, which will provide hope for the future for all sarcoma patients.